
In addition, the recommended risk-based approach is in line with the application of the European EMA and US FDA regulations governing computer system validation, Annex 11 and 21 CFR Part 11, respectively. However, the framework described in this guidance document provides a comprehensive approach to the validation of computer systems that are generally accepted within the industry. GAMP COP (Community of Practice) is a pharmaceutical professionals’ forum that ensures continued development and adoption of best practices in the field. Therefore, it is not mandatory to follow this methodology. GAMP guidelines are used heavily by the pharmaceutical industry to ensure that drugs are manufactured with the required quality.
The strategies defined in GAMP5 are guidelines, not regulations.

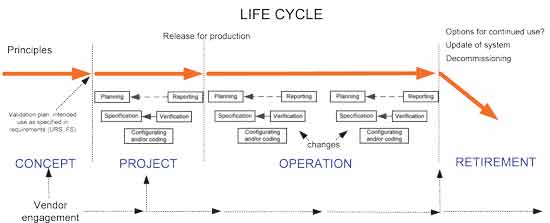
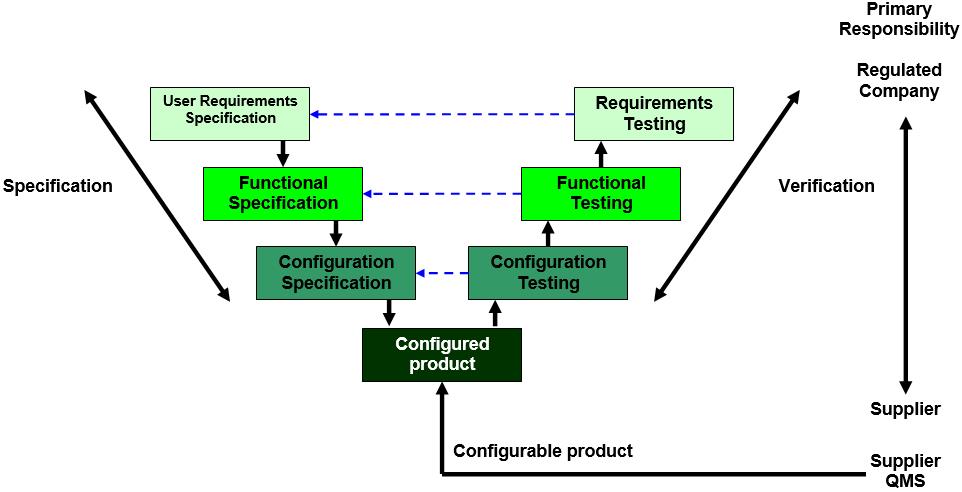
The system categorization helps guide the drafting of system documentation. The ISPE GAMP5 guidance is aligned to the ICH Q9 guidance and both suggest the use of risk assessment as the key input to deciding the extent and effort for validation. Simply put, GAMP5: A Risk-Based Approach for Compliant GxP Computerized Systems provides a framework for a risk-based approach to computer system validation where a system is evaluated and assigned to a predefined category based on its intended use and complexity. the GAMP 5 guidance on the risk-based approach and its implementation.
GAMP5 GUIDELINES SOFTWARE
Consequently, it is important to take a holistic approach to applying GAMP 5 by incorporating the principles in the relevant processes. Introduction GAMP5 In this article we will share examples of GAMP software categories that are related to The Good Automated Manufacturing Practices (GAMP5) Guide for Validation of Automated Systems in Pharmaceutical Manufacture. GAMP® 5 defines Software Categories that may be used along with risk assessments and supplier assessments to develop a suitable and streamlined validation strategy for your software application. This document is published by an industry trade group called the International Society of Pharmaceutical Engineering (ISPE) based on input from pharmaceutical industry professionals. The guidelines not only apply for validation, but also for various processes within your organization as risk management, supplier relation, and system maintenance. Within the validation world, one of the most adopted guidelines is GAMP 5.
Generally, GAMP5 refers to a guidance document entitled GAMP®5: A Risk-Based Approach to Compliant GxP Computerized Systems. GAMP stands for Good Automated Manufacturing Practice.


 0 kommentar(er)
0 kommentar(er)
